Wavelength of Light :Newton provided evidence that colour is a property of light. Therefore, knowledge of light is important in order to comprehend colour. Light is an example of electromagnetic radiation and shares characteristics with both waves and particles. It may be pictured as a stream of tiny energy packets that are emitted in a wave motion at various frequencies. Any given light beam has a certain frequency, wavelength, and energy value attached to it. Hertz units are frequently used to describe frequency, which is defined as the quantity of waves passing a fixed point in space in a unit of time (1 Hz = 1 cycle per second).
What is the Wavelength?
In physics, a periodic wave’s wavelength is defined as its period. It is the length that the wave repeats its form over. It measures the separation between successive similar values of the same phase. For instance, two zero-crossings, crests, or troughs. It is a characteristic of both moving and stationary waves. Frequency is the wavelength’s inverse. Typically, we use the Greek letter lambda () to represent a wavelength.
The wavelength is inversely related to the frequency of the wave when one anticipates a sinusoidal wave travelling at a fixed wave speed. In general, waves with higher frequencies have a shorter wavelength than waves with lower frequencies, and vice versa. The medium through which a wave travels—such as a vacuum, air, or body of water—determines its wavelength.
The measured separation between two equal spots on two succeeding waves is known as a wavelength. Wavelength may be calculated by comparing the distance between two adjacent crests or troughs. Transverse waves, which move perpendicular to the direction in which photons oscillate, are the type of light that travel across empty space. The units for wavelength are either nanometers (nm) or micrometres (m), and the symbol for wavelength is (lambda). In the case of waves, the wavelength may be stated as follows.
λ = c/f
Or, with respect to particles,
E = hf, and
λ is the light’s wavelength
c is the speed of light in a vacuum or open space.
f is the frequency at which the light wave’s photons oscillate.
E is the energy of the light wave
h is the Planck’s constant (6.64 x 10-34 joule/ second)
What is Visible Spectrum?
The part of the electromagnetic wave that can be observed by human vision is known as the visible spectrum. The visible spectrum in the electromagnetic spectrum spans from the infrared to the ultraviolet regions. Between the infrared and ultraviolet wavelength ranges is where the visible light falls. From 400 nanometers (violet) to around 700 nanometers, the human eye can sense light (red). Other electromagnetic radiations are either outside the range of biological function or are too tiny or huge to be detected by the human eye.
These waves may be compared to the colours of the rainbow, where each colour has a unique wavelength. The corona, the topmost layer of the sun, is also visible in visible light.
Wavelenght of Visible Light
Even while all electromagnetic energy is light, humans can only perceive a small fraction of it, which we refer to as visible light. Our eyes’ cone-shaped cells serve as receivers tuned to the wavelengths in this condensed band of the electromagnetic spectrum. Other parts of the spectrum contain energy wavelengths that are either too big or too little for our biological senses to perceive.
The wavelengths of visible light split into the rainbow’s hues as it passes through a prism because each colour has a unique wavelength. Red has a wavelength of around 700 nanometers, whereas violet has a wavelength of about 380 nanometers.
Visible Spectrum of Light
| Color | Wavelength (in nm) | Frequency (in THz) |
| White | 750 – 400 nm | 790 – 400 |
| Red | 750 – 610 nm | 480 – 405 |
| Orange | 610 – 590 nm | 510 – 480 |
| Yellow | 590 – 570 nm | 530 – 510 |
| Green | 570 – 500 nm | 580 – 530 |
| Blue | 500 – 450 nm | 670 – 580 |
| Indigo | 450 – 425 nm | 600 – 670 |
| Violet | 425 – 400 nm | 700 – 790 |
- White light: The wavelength of white light is 400–750 nm. The light spectrum is created by the passage of white light through a prism-like crystal structure by the refraction of various wavelengths at various angles.
- Red Light: Red light has a wavelength that falls between 750 and 610 nanometers. When the incoming sunlight is poorly scattered by the Earth’s atmosphere during sunsets and sunrises, the sky may seem crimson.
- Orange Light: The wavelength range for orange lights is between 610 and 590 nm. Similar to red, orange may also be seen during sunrises and sunsets.
- Yellow Light: The wavelength of yellow light ranges from 590 to 570 nm. Low-pressure sodium lights emit this yellow light.
- Green Light: The wavelength of green light ranges from 570 to 500 nanometers. In grass and leaves, it is plain to see. Grass appears green because it reflects the green wavelength and absorbs all other wavelengths.
- Blue Light: Between the wavelengths of 500 and 450 nm is blue light. Because the atmosphere scatters shorter wavelengths, it also scatters the wavelength that gives blue its colour. When we glance up, the sky seems blue because of this.
- Violet and indigo lights appear to be quite like since their wavelengths are roughly comparable in value.
The Relationship Between Frequency and Wavelength
Frequency and wavelength are intimately related, particularly in the context of light. Frequency is determined by the number of waves that pass through a single place over a particular period of time, whereas wavelength is the distance between two successive troughs or crests. The relationship between wavelength and frequency is inverse, therefore the frequency decreases as the wavelength increases. Since more troughs and crests pass through the given spot when the wavelength tends to be short, the frequency tends to be greater. On the other hand, frequency is often lower when the wavelength has a longer journey.
The measurement of colour
The term “colorimetry” refers to the measuring of colour. In this discipline, several instruments are employed. The most advanced analyse light according to the amount of energy contained at each spectral wavelength, called spectrophotometers. The emittance curves for light sources and the reflectance curve of the paint pigment known as emerald green are examples of typical spectrophotometer results.
A given spectral energy distribution’s colour is challenging to characterise. It is vital to represent colour measurements in a perception-related manner since the eye can only distinguish one colour for each given energy distribution. There are several systems, some of which are described here.
Wavelength of light in angstroms
Light’s wavelength value is expressed in angstroms. J Angstrom, a Swedish scientist, gave the thing its name. A value of one Angstrom corresponds to 0.1 nanometers or 1010 metres. This angstrom is used to express the different light wavelengths, including visible light, X-rays, gamma rays, and ultraviolet light (UV). Visible light wavelengths were once expressed in angstroms, but this is no longer a common practise. Everything that the human eye can see has wavelengths between 4500 and 7000 angstroms. The VIBGYOR colours are shown here, along with their angstrom-based wavelengths.
- 7000 angstroms is the wavelength of red light.
- 6200 angstroms is the wavelength of orange light.
- 5600 angstroms is the wavelength of yellow light.
- 5150 angstroms is the wavelength of green light.
- 4700 angstroms is the wavelength of blue light.
- 4400 angstroms is the wavelength of indigo light.
- 4100 angstroms is the wavelength of violet light.
Thus, there are several ways to define light, and electromagnetic radiation is one accepted definition in physics. Additionally, light can have many representations depending on the VIBGYOR. The varied hues were created by this VIBGYOR.
Wavelenght of Red Light
The electromagnetic spectrum, which includes the whole range of visible and invisible light that travels from the sun to earth, has a wavelength for red light between 620 and 750 nanometers.
What is the Best Wavelength for Red Light Therapy?
What wavelengths are used in red light and red light therapy? Joovv, an efficient red light treatment device, uses wavelengths in the mid-600 nm range. In peer-reviewed clinical research, these wavelengths have been examined and evaluated, and it has been determined that they are safe and advantageous for human health in a number of areas, including skin, pain, and general physical performance.
What Distinguishes Red Light from Near Infrared Light (NIR)
Red light can promote better skin health and healing since it is easily absorbed by surface tissues and cells.
Since near infrared light is invisible to the human eye and may reach deeper tissues, it may promote faster healing and less inflammation.
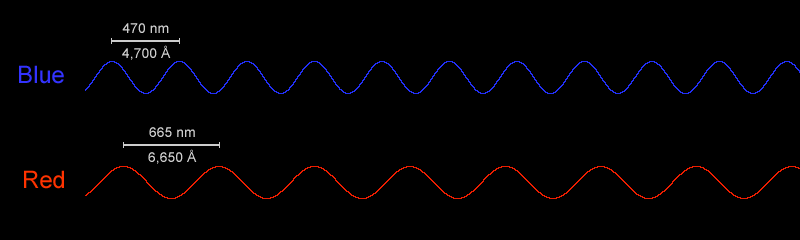
the wavelengths of red and blue light
Blue light has a higher frequency (or shorter wavelengths) than red light, with wavelengths in the 450–495 nanometer range. When you consume too much blue light at night via devices and artificial lighting, it can disrupt your sleep and give you headaches because it is also considerably brighter than red light.